Lithium Solid State Batteries
Since the early 1970s, polymers have been considered as promising lithium-ion battery materials. However, studies of these substances have yielded disappointing results. While some PEO-based SPEs are effective, others are not. The main drawback of PEO-based SPEs is their low ion conductivity at room temperature. In addition, they are regarded as insulators because they contain 30% to 50% liquid solvent.
In lithium-ion batteries, solid polymer electrolytes are composed of a mixture of metallic Li or polyethylene oxide. The metallic lithium salts are more efficient because of their higher specific energy, but they are less stable than polyethylene glycol. Both materials are dispersed and have different molecular weights. These ionic conductivity properties are important for the operation of lithium-ion batteries.
A major challenge for developing lithium-ion batteries is improving their performance. To enhance their energy storage capacity, they must improve their ionic conductivity. Polymer electrolytes should not only be liquid but also be free of any crystalline structures. Alternatively, they should be transparent or contain a thin layer of amorphous silicon. In addition to using a solid electrolyte, polymer-based electrolytes should be made of two- to three-dimensional layers to increase their efficiency.
Manganese Market Analysis by Martin Kepman CEO of Manganese X Energy Corp
Polymer electrolytes are the key to lithium batteries. The choice of an electrolyte is crucial for the performance of a lithium-ion battery. Several types of composite polymer electrolytes exhibit excellent interface compatibility and high ion conduction. In addition to the ionic conductivity, polymer-based solid-state batteries may also be able to improve safety.
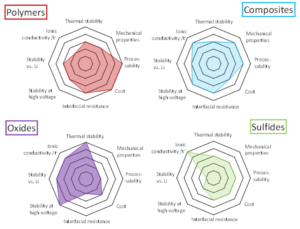
The Role of Polymers in Lithium Solid State Batteries
The role of polymers in lithium solid-state batteries depends on the nature of the lithium electrolyte. For example, polyethylene glycol is the preferred polymer for lithium-ion batteries because of its high electrostatic and ion-transport capacity. Besides PEO, polycarbonates are also known as functional polymers. Further, these materials can be tuned to promote SPE self-organization, limit transference, and avoid dendrites.
The role of polymers in lithium solid-state batteries is vital to the overall performance of the cells. As the primary material for the cells, polyethylene glycol has been accepted for its high ion-conductivity and high ability to reduce crystallinity. This material is also beneficial for the electrochemical reactions that occur between the lithium and polymer. The main drawbacks of traditional liquid-based liquid-state batteries are their poor ion-transporting capacity.
Polymers are useful as an electrolyte. They are cheap, conformable, and have low electrochemical potential. While polymers are generally not a good choice for the anode, they are often suitable as electrodes. Hence, lithium-ion batteries are considered to be a good alternative for electric vehicles. The key is to combine these two materials. But what about the anode?



